Faculty Research Activity
- The size and breadth of our own faculty enable unique, internal research partnerships.
- Our faculty is connected nationally and internationally through leadership in the American Society of Hematology, the American Society of Clinical Oncology, the World Marrow Donor Association, the National Comprehensive Cancer Network, the Breast Cancer Research Foundation and others.
- The majority of our faculty members have joint appointments in scientific research departments at the University of Washington or the Fred Hutchinson Cancer Center.
- A quarter of our faculty members have Ph.D.s, encouraging cross-fertilization with related scientific fields.
Clinical trials
Clinical trials are the critical link between lab research and approved therapies.
- The majority of our faculty members are involved in clinical trials, often in collaboration with many other institutions.
- Our faculty lead Fred Hutch’s Phase 1 Program to develop and implement the most innovative clinical trials—the first step in testing new therapies in humans.
Select Research Topics
Genomically-driven, personalized medicine for treatment of blood diseases and cancer
We combine cell biology, molecular biology, chemistry, engineering, nanomedicine and bioinformatics to develop new gene therapies. An example is gene editing—“molecular scissors”--for many diseases caused by defective genes, including cancers.
Outcomes of cancer therapy
Through the Hutchinson Institute for Cancer Outcomes Research (HICOR), our faculty use outcomes data to improve prevention, detection, and treatment. HICOR also addresses the financial impact of cancer care and tests methods of helping patients and families reduce and manage debt.
Bone marrow and stem cell transplantation
Dr. E. Donnall Thomas received the Nobel Prize for pioneering this therapy. Recently our innovations have made transplants accessible more broadly—to patients from minority and marginalized communities and to older patients.
- We led the international development of marrow banks and the campaigns to have a greater variety of potential donors.
- Our faculty developed “mini-transplants” for fragile or older patients who would not be eligible for traditional transplants.
- We continue to refine identification of the donor match points (HLA types) to maximize the possibilities of a successful match.
- Our team pioneered umbilical cord blood transplant and, through cell expansion, made it clinically practical, extending transplants to patients without a traditional donor match.
- Our risk-assessment model for acute myeloid leukemia informs therapeutic choices, especially for older or marginalized patients.
Ensuring healthy new blood cells
We explore cellular and molecular mechanisms that permit quick and accurate creation of mature red cells and the glitches that result in anemia or myelodysplastic syndrome. We are testing the potential of small molecule competitive inhibitors that ensure healthy cells.
Tumor vaccines
In conjunction with the UW Cancer Vaccine Institute, basic research has progressed to active clinical trials in vaccine therapy to prevent cancer recurrence.

Cell-based immunotherapy
Immunotherapy uses the body’s own immune system to find and eradicate tumors.
- Our teams developed some of the first CAR T-cell therapies, engineering patients' own T cells to target cancer cells.
- Our faculty led research on checkpoint inhibitors that teach our bodies’ immune cells to target and block cancer’s pathway.
Antibody immunotherapy
Antibodies target cancer cells, sparing the normal, healthy cells in the body, greatly reducing side effects of radiation or drugs.
- We explore a variety of monoclonal antibodies that bind to cancer cells to interfere with tumor growth.
- Radioimmunotherapy, pioneered by our faculty, pairs radioactivity with targeted antibodies to deliver radiation directly to cancer cells.
- Antibodies are combined with drugs to ensure that the drug toxicity is limited to the cancer cells.
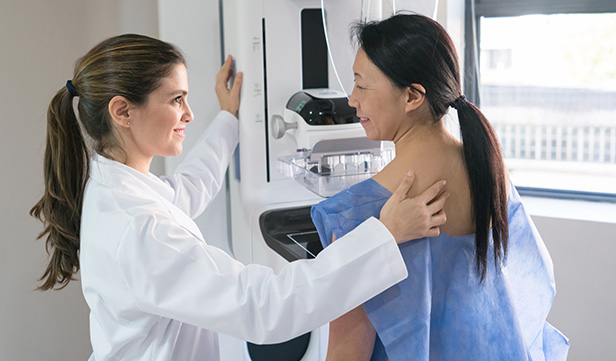
Prevention of recurrence in breast cancer
Our faculty examine the role of hormones in breast cancer growth, how estrogen receptor genes are regulated by epigenetic factors, and how estrogen deprivation and other therapies trigger breast cancer cells to kill themselves through apoptosis, or programmed cell death.
Identification of BRCA mutations in metastatic prostate cancer
By understanding the relationship between mutations and prostate cancer, genetic screening identifies the most appropriate treatment for an individual patient. National collaboration through The Cancer Genome Atlas informs this research.
hematology research funds




